Which Statement Describes an Arrhenius Acid
It is used in situations that involve bases that do not produce hydroxide ions. AA substance that increases H3O concentration when it is dissolved in water.

Acidic Solution Easy Science Solutions Flashcards Biology Notes
It has a wider range of applications than the Bronsted-Lowry interpretation.

. CA compound that donates protons. An Arrhenius acid is a substance that dissociates in water to form hydrogen ions H. An Arrhenius acid tastes sour.
It is limited to situations that involve aqueous solutions or specific compounds. C Bronsted-Lowry base is a proton acceptor and an Arrhenius base is a hydroxide. You must familiar with automobile battery which contains sulphuric acid is a key part of acid rain.
A substance that conducts an electrical current when dissolved in water is called. Hydrogen chloride HCl is classified as an Arrhenius acid because it produces. 3 The only positive ion in solution is H.
2 The only negative ion in solution is HCO3-. What is the Arrhenius definition of an acid. An Arrhenius acid reacts with a base to produce a salt and water.
-Acids are H acceptors and bases are H donors. H ions in aqueous solution. Which of the following statements best describe a Bronsted Lowry base and an Arrhenius base.
In aqueous solutions the hydrogen ions react with water molecules to from hydronium ions H3O. It produces hydroxide ions in a solution. An Arrhenius acid accepts H1 during reaction.
Which of the following statements does not accurately describe a characteristic property of an Arrhenius acid. An Arrhenius acid turns red litmus blue. -Acids are H donors and bases are H acceptors.
BA substance that increases OH- concentration when it is dissolved in water. 4 The only positive ion in solution is NH4. Correct answer - Which statement describes an arrhenius acid.
Hence the correct statement is arrhenius acid produces hydrogen ions in solution. Which of the following statements describes an Arrhenius acid. Arrhenius Theory of Acid and Base.
-Acids and bases are both H acceptors. According to Arrhenius concept an acid is defined as a substance which donates hydrogen ions in water and a base is defined as a substance which donates hydroxide ions when dissolved in water. An Arrhenius acid is a substance that increases the concentration of hydronium ion in water.
Household drain cleaners often contain strong bases which are. Which statement best describes the solution produced when an Arrhenius acid is dissolved in water. According to the Bronsted Lowry conjugate acid-base theory an acid is defined as a substance which donates protons and a base is defined as a substance which accepts protons.
Hence the correct statement is arrhenius acid produces hydrogen ions in solution. It has a wider range of applications than the Lewis and Bronsted-Lowry interpretations. An Arrhenius acid turns red litmus blue.
In neutralization reaction an Arrhenius acid and base will react together to form salt and water. Which statement describes an alternate theory of acids and bases. An Arrhenius acid refers to a substance that dissociate in water to form protons or hydrogen ions.
A Bronsted-Lowry base is a proton donor and an Arthenius base is a hydroxide donor b Bronsted-Lowry base is a proton donor and an Arrhenius base is a proton donor. Acids are H donors and bases are H acceptors. When an Arrhenius acid dissolves in water the only positive ion in the solution is.
Which characteristic best identifies an Arrhenius base. According to the Lewis concept an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs. The base is a substance that ionizes OH ion by dissolving in the aqueous solution.
Decreases by a factor of 1000. 1 The only negative ion in solution is OH-. The acid-base reaction is considered a type of neutralization reaction where the acid and base react to yield water and a salt.
It is a proton donor. The correct answer is not given as an option. An Arrhenius acid donates OH-1 during reaction.
The concentration of OH- ions is high in the solution. It is a proton acceptor. According to the Lewis concept an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs.
An Arrhenius acid reacts with a base to form H2O. Which statement describes the Arrhenius interpretation of acids and bases. According to Arrhenius theory acid is a substance that gives H ion on dissolving in the aqueous solution.
The acid usually increases the number of hydrogen ions in aqueous solution. -Acids and bases are both H donors. Acidity and alkalinity describe the concentration of hydrogen ions acidity and hydroxide ions alkalinity.
According to the Arrhenius description of acids and bases the water molecule consists of a proton and a hydroxide ion. Which statement describes the Arrhenius interpretation of acids and bases. DA compound that accepts protons.
It increases the concentration of H ions in the solution.

Solved What Characteristics Do All Arrhenius Acids Have In Common An Arrhenius Acid Has Oh In Its Formula And Dissociales In Water To Yield H 0 An Arrhenius Acid Has Hin Its Formula
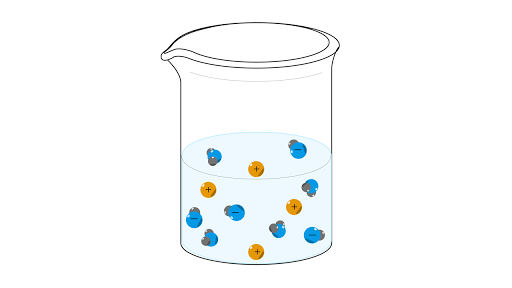
Arrhenius Acids And Bases Article Khan Academy

Question Video Identifying The Definition Of An Arrhenius Acid Nagwa

Arrhenius Acids And Bases Video Khan Academy

𝙿𝚒𝚗𝚝𝚎𝚛𝚎𝚜𝚝 𝚕𝚞𝚑𝚑𝚋𝚡𝚡𝚋𝚢𝚢𝚢𝚢 Video Science Notes Physics Notes School Organization Notes
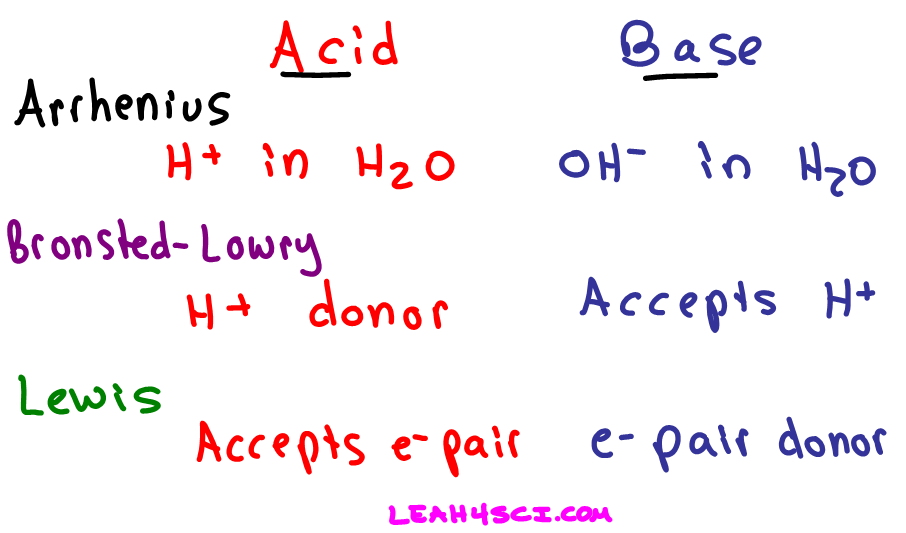
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry

Molecular Orbital Diagram Wikipedia The Free Encyclopedia Diagram Molecular Science Chemistry

The Following Venn Diagram Shows The Similarities And Differences Between Acids And Bases Ideal For Cl Venn Diagram Venn Diagram Examples Chemistry Worksheets

Acid Base Theories Arrhenius Bronsted Lowry Acids Chemtalk
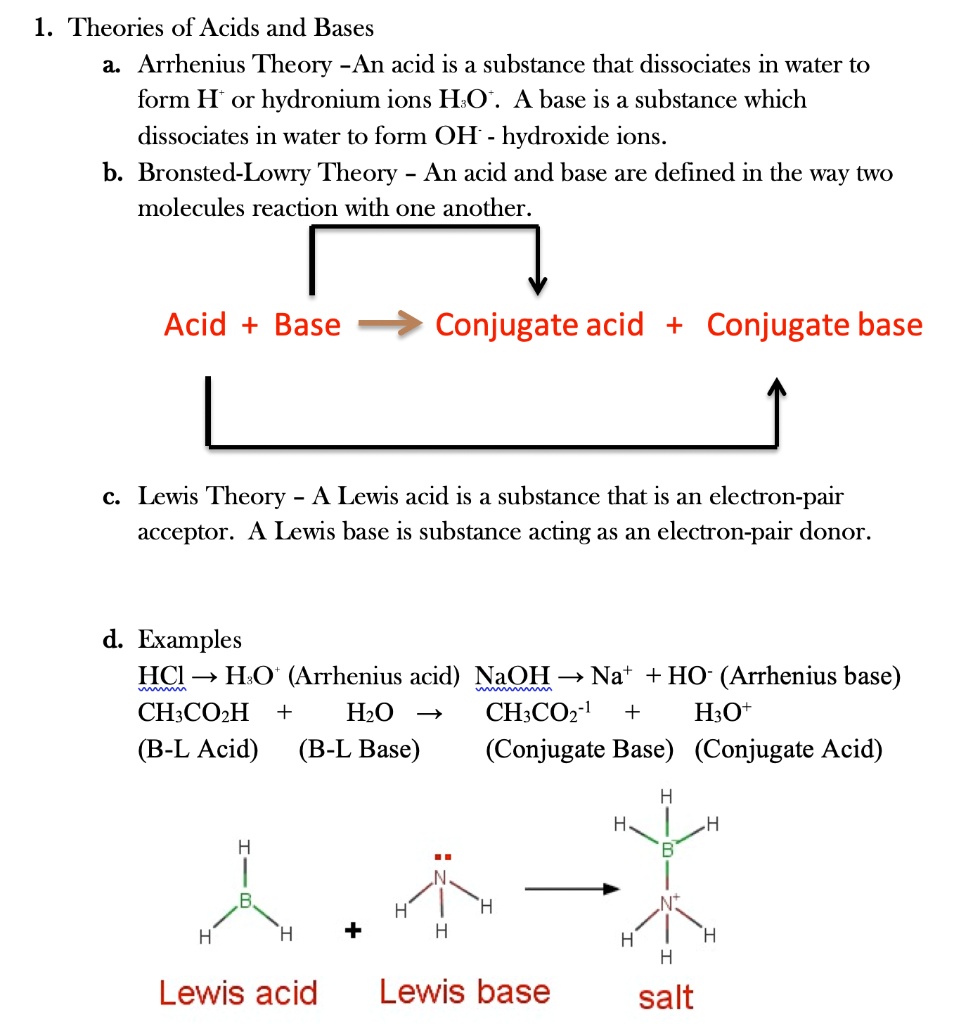
Solved 1 Theories Of Acids And Bases Arrhenius Theory An Acid Is A Substance That Dissociates In Water To Form H Or Hydronium Ions Ho A Base Is A Substance Which Dissociates

Bond Comparison Bond Scale Restaurant Visual Learning Covalent Bonding Chemistry
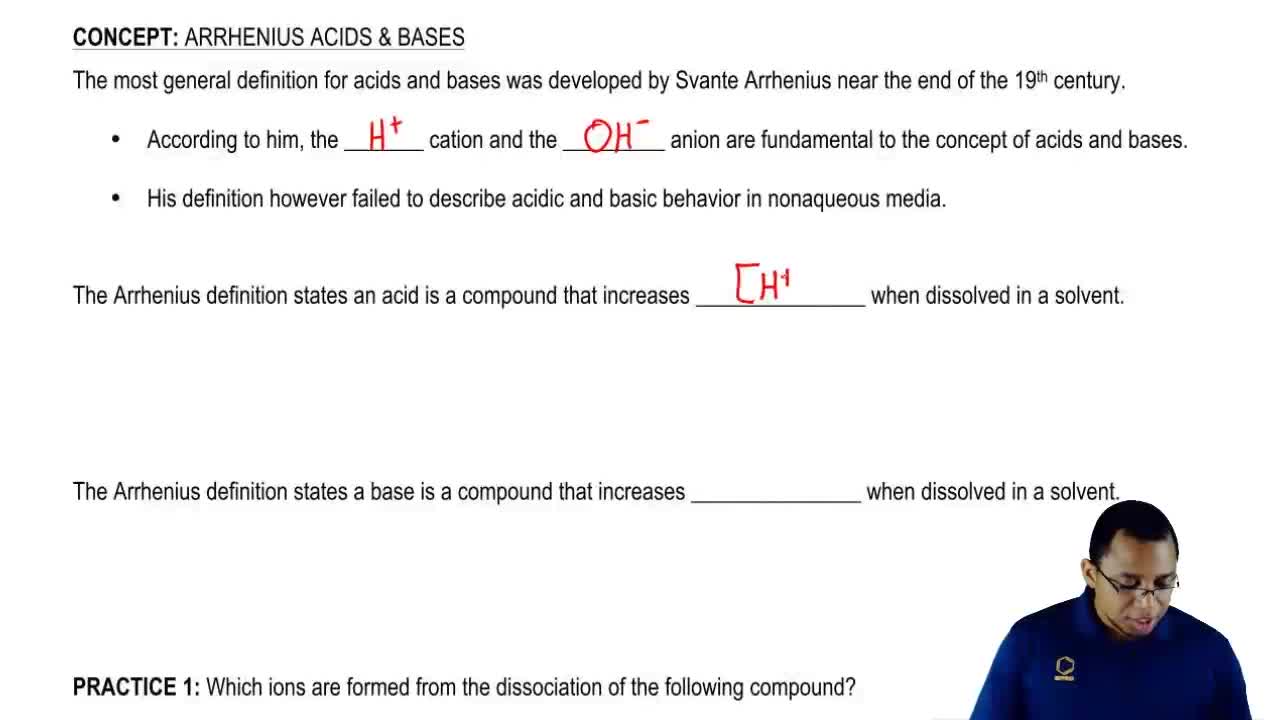
Arrhenius Acid And Base Chemistry Video Clutch Prep

đại Cương Carbohydrate Www Docsachysinh Com Chemistry Math Ebook

Electronegativity Bond Scale Surfguppy Chemistry Made Easy Visual Learning Covalent Bonding Chemistry Visual Learning

Pin On Acids Bases And Solutions

Rates Of Reaction The Effects Of A Reactions Graphing Energy Activities

Chemistry Worksheet Calculating Wavelength Visible Light Quantum Numbers Chemistry Worksheets Super Teacher Worksheets Teaching Chemistry

Chemical Reaction And Changes In Science Full Guided Science Lesson Bundle Science Lessons Elementary Science Lessons Science Lesson Plans

Protein Tertiary Structure Wikipedia Learn Biology Structural Biology Chemical Kinetics
Comments
Post a Comment